



The nasal valve is the narrowest area of the nasal airway accounting for two-thirds of nasal resistance. But as Poiseuille’s Law states, airflow is proportional to the 4th power of the radius, so small changes can deliver exponential improvements in nasal breathing.
VivAer is an office-friendly, incisionless, non-invasive procedure that offers patients a solution from NAO. Compact and portable, the Aerin® Console and VivAer Stylus fit seamlessly into any clinic, office, or hospital environment. VivAer is a convenient and efficient procedure option to integrate into your practice.

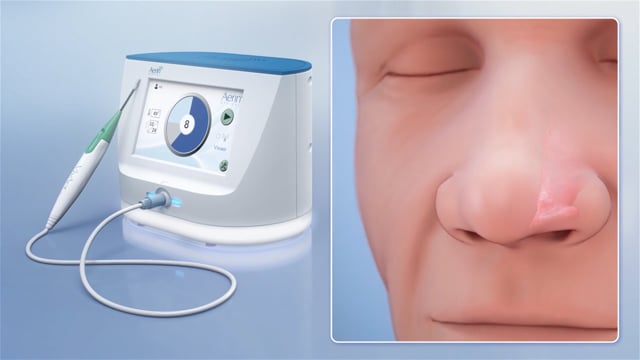
The low profile VivAer Stylus employs temperature-controlled radiofrequency (RF) technology, optimized for the nasal airway. Once the Stylus is inserted into the nose, the Aerin System automatically maintains the tip’s target temperature for therapeutic benefit while sparing surrounding tissue.
VivAer enables you to effectively tackle many of the core causes of nasal airway obstruction with one procedure:
Results from a pivotal follow-up study2,3

92.9% of patients reported improved breathing through their nose during exercise or exertion.2

>60% of patients reported using fewer or less frequent oral medications, nasal sprays and nasal breathing strips.2
0 serious adverse events related to the device and/or procedure were observed in the study1
All 5 components of the NOSE Scale (nasal congestion, nasal blockage, trouble breathing, trouble sleeping, and being unable to get enough air during exercise) demonstrated improvement.

Study design: Prospective, non-randomized, multi-center, extended follow-up study to determine if safety and efficacy results achieved at the 6-month timepoint for the VivAer® pivotal trial would be sustained at the 48-month timepoint.
Patients: A total of 29 subjects who were treated with the VivAer® Stylus in the 50-subject TP258 interventional study were followed up to 48 months.
Primary endpoint:
Change from baseline NOSE score to 12-, 18-, and 24-months post-procedure; number and percentage of participants with positive response on QoL assessment items at 12-, 18-, and 24-months post-procedure; change from baseline NOSE score to 36-, 48-, and 60-months post-procedure; number and percentage of participants with positive response on QoL assessment items.



Jamie
VivAer Patient
“Everyday when I wake up, rather than feeling congested like I had been before, I feel good as new. It really feels amazing.”
References:

The VivAer Stylus plugs into the compact and portable Aerin® Console. The console may also be used with the RhinAer® Stylus to deliver comprehensive, durable procedure for chronic rhinitis.
RhinAer.com